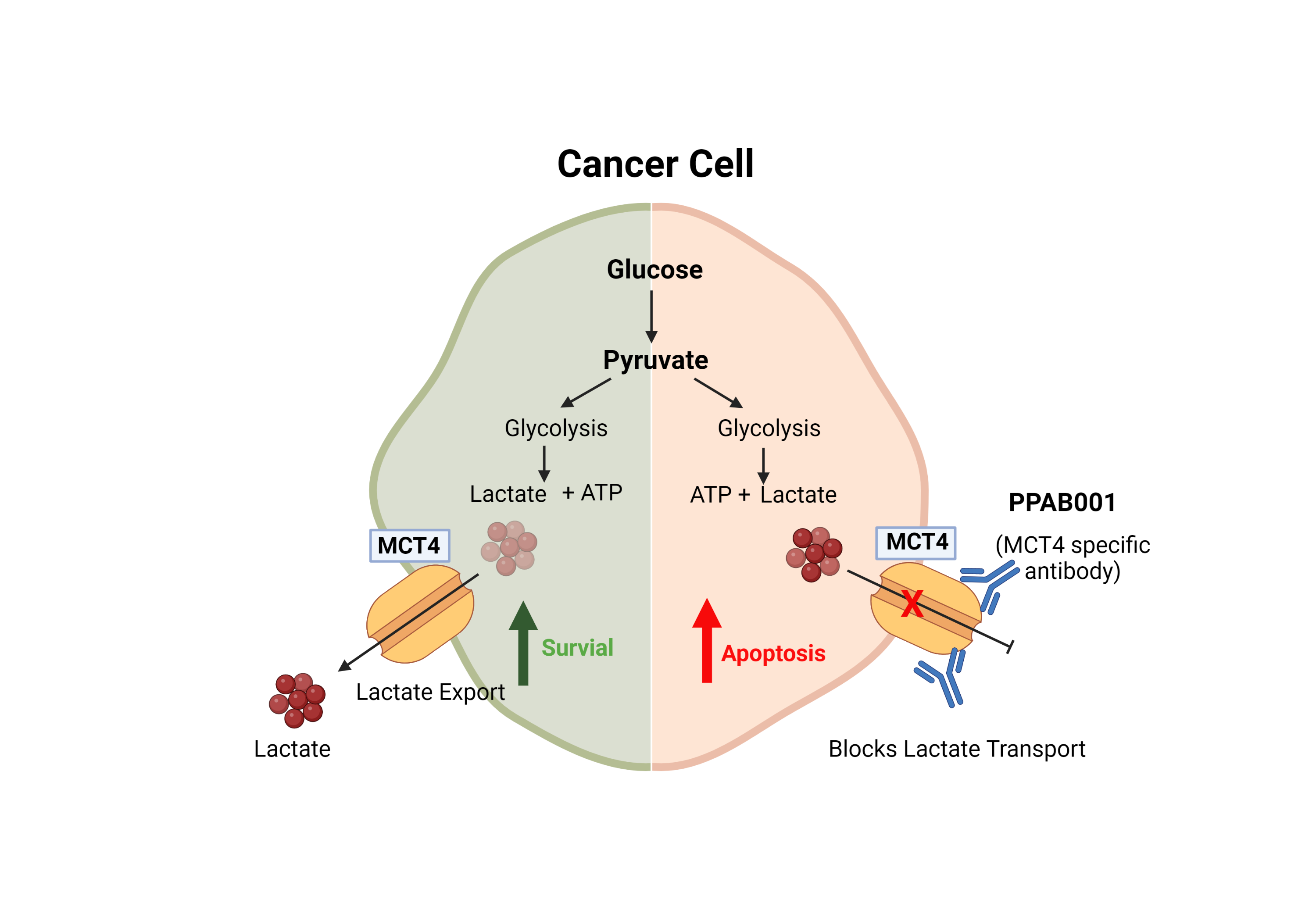
Monocarboxylate transporter 4 (MCT4) is highly expressed in many malignant cancer cells that preferentially metabolize glucose anaerobically instead of aerobically due to the Warburg effect. MCT4 has long been proven to be a promising therapeutic target in many cancer types. Yet, effective MCT4-targeted therapeutic antibodies are not available due to technological challenges involved with developing functional antibodies that specifically blocks MCT4's lactate transport activity.
At PharmaPlanter Technologies, using our proprietary antibody discovery platform, we have developed a panel of antibodies that target MCT4 (denoted as PPAB001). PPAB001 has shown specific activities that oppose MCT4’s lactate transportation. This project is currently in the preclinical drug development phase.
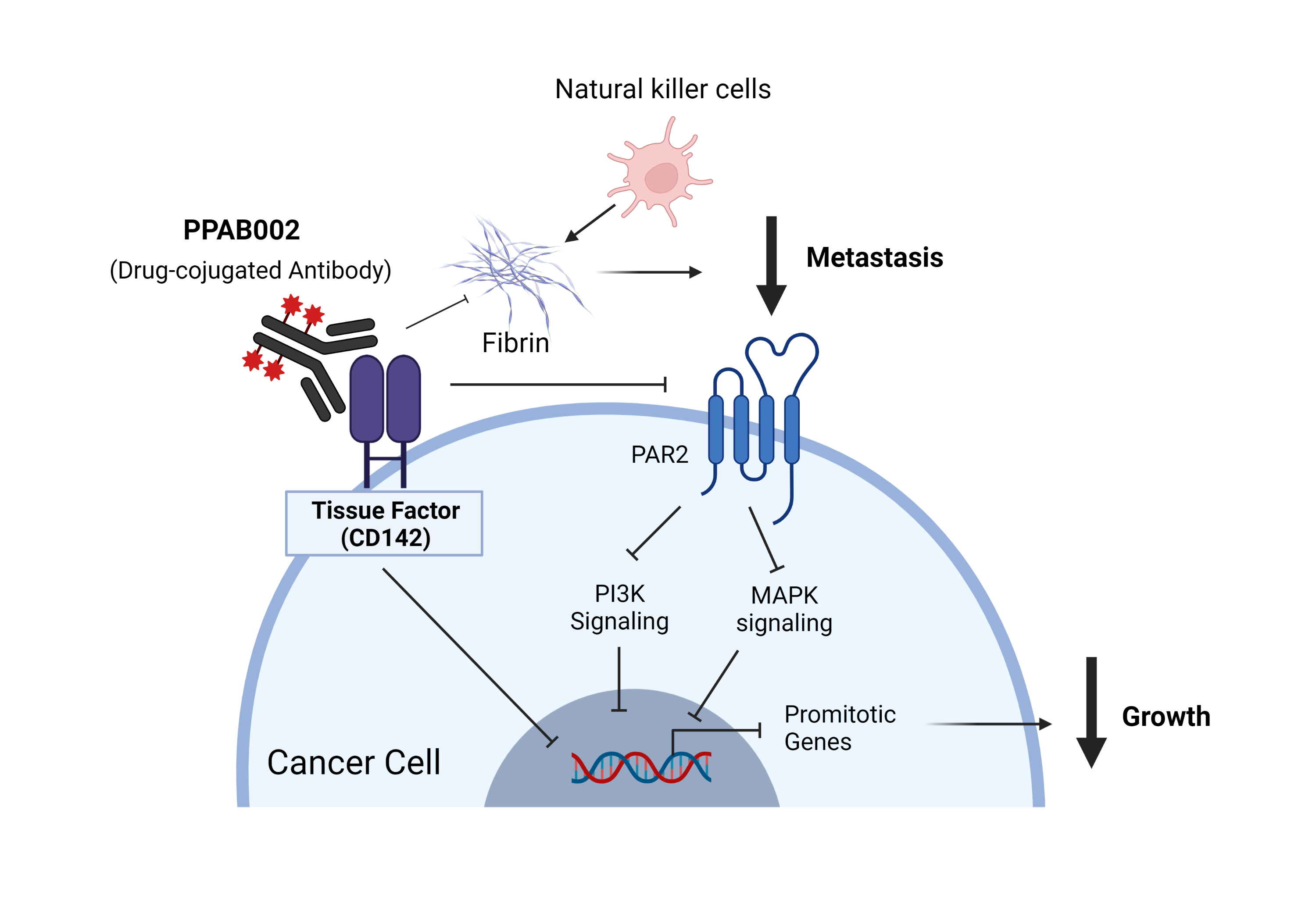
Tissue factor (TF), also known as CD142, is a transmembrane glycoprotein that activates the coagulation cascade and triggers blood clotting. However, TF is over expressed by many tumour cells and play a key role in various pathologic processes such as thrombosis, metastasis, tumour growth, and tumour angiogenesis.
So, in collaboration with Shanxi Biological Research Institute and iProgen Biotech, we have developed a novel antibody-drug conjugate that targets TF (denoted as PPAB002). This drug candidate shows anti-tumour activity and dramatically reduces procoagulant microparticles in the circulatory system. This project is currently in the preclinical phase of drug development.
Strategies targeting CD47 have gained popularity in cancer immunotherapy. At PharmaPlanter Technologies, we have developed a series of novel CD47-based therapeutics, including a protease-activated antibody-drug conjugate that targets CD47 (denoted as PPAB003) and a bispecific antibody fusion protein that targets both CD47 and CD24 (denoted as PPAB004).
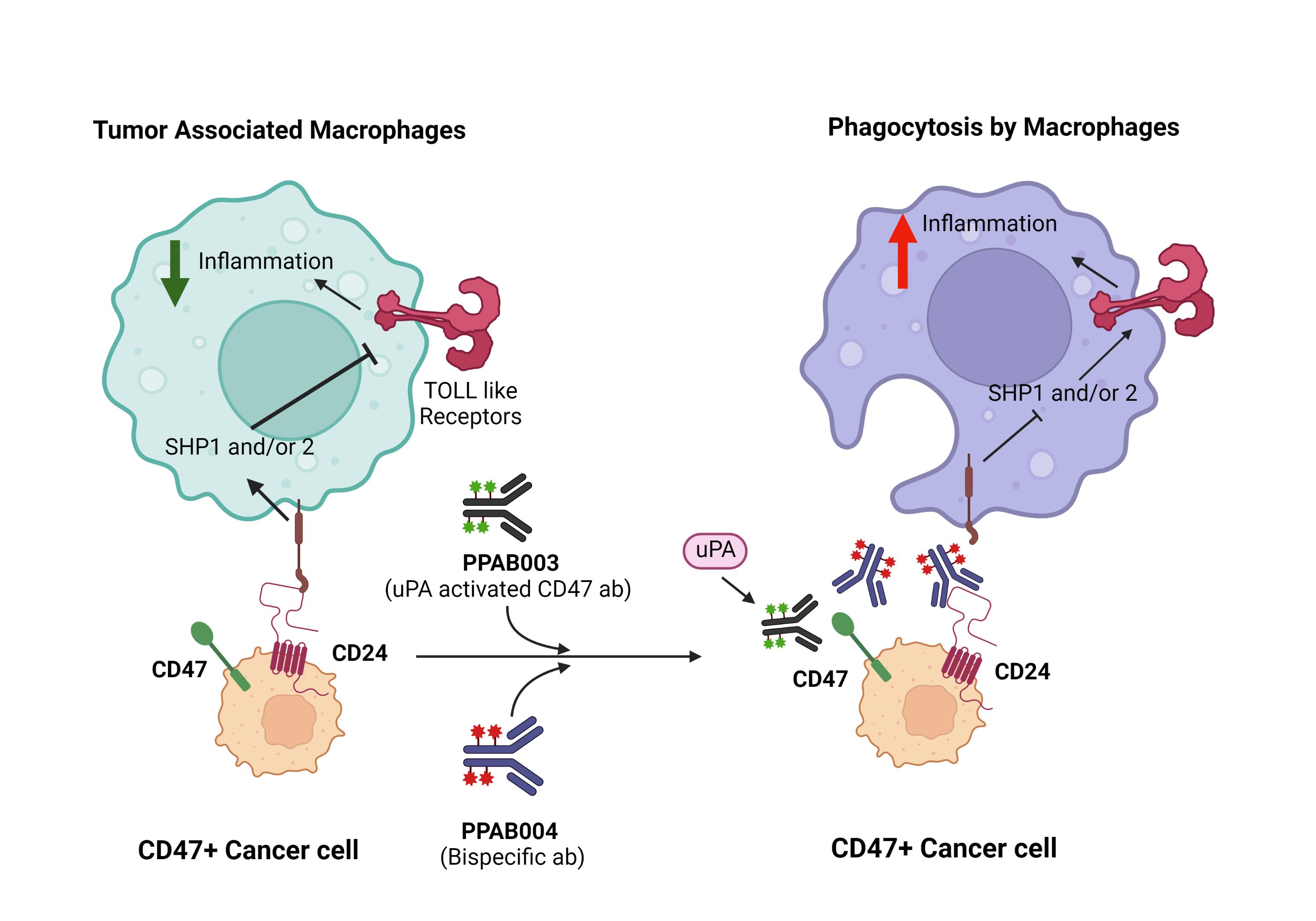
It is well known that the serine protease known as urokinase-type plasminogen activator (uPA) is up-regulated in a variety of human carcinomas. PPAB003 is designed as a uPA-activated antibody drug that selectively targets CD47-positive tumor cells and exerts greater anti-tumor activity compared to traditional anti-CD47 antibody therapeutics.
Recently, CD24 has been verified as a highly expressed, anti-phagocytic signal in several cancers. In humans, CD24 has been known to interact with Siglec-10 on innate immune cells to dampen damaging inflammatory responses to infection, sepsis, liver damage, and chronic graft-versus-host disease. The binding of CD24 to Siglec-10 elicits an inhibitory signaling cascade that is mediated by Src homology region 2 domain-containing phosphatases 1 and/or 2 (SHP1 and/or 2). Inhibition of CD24 by PPAB004 thus activates the signaling cascade.
These phosphatases are associated with the two immunoreceptor tyrosine-based inhibition motifs in the cytoplasmic tail of Siglec-10, thereby having the ability to block Toll-like-receptor-mediated inflammation and the cytoskeletal rearrangement required for cellular engulfment by macrophages. Consequently, a bispecific antibody targeting both CD47 and CD24 will further activate of the innate immune system and enhance phagocytosis. Currently, both PPAB003 and PPAB004 therapeutics are actively undergoing the preclinical phase of drug development.
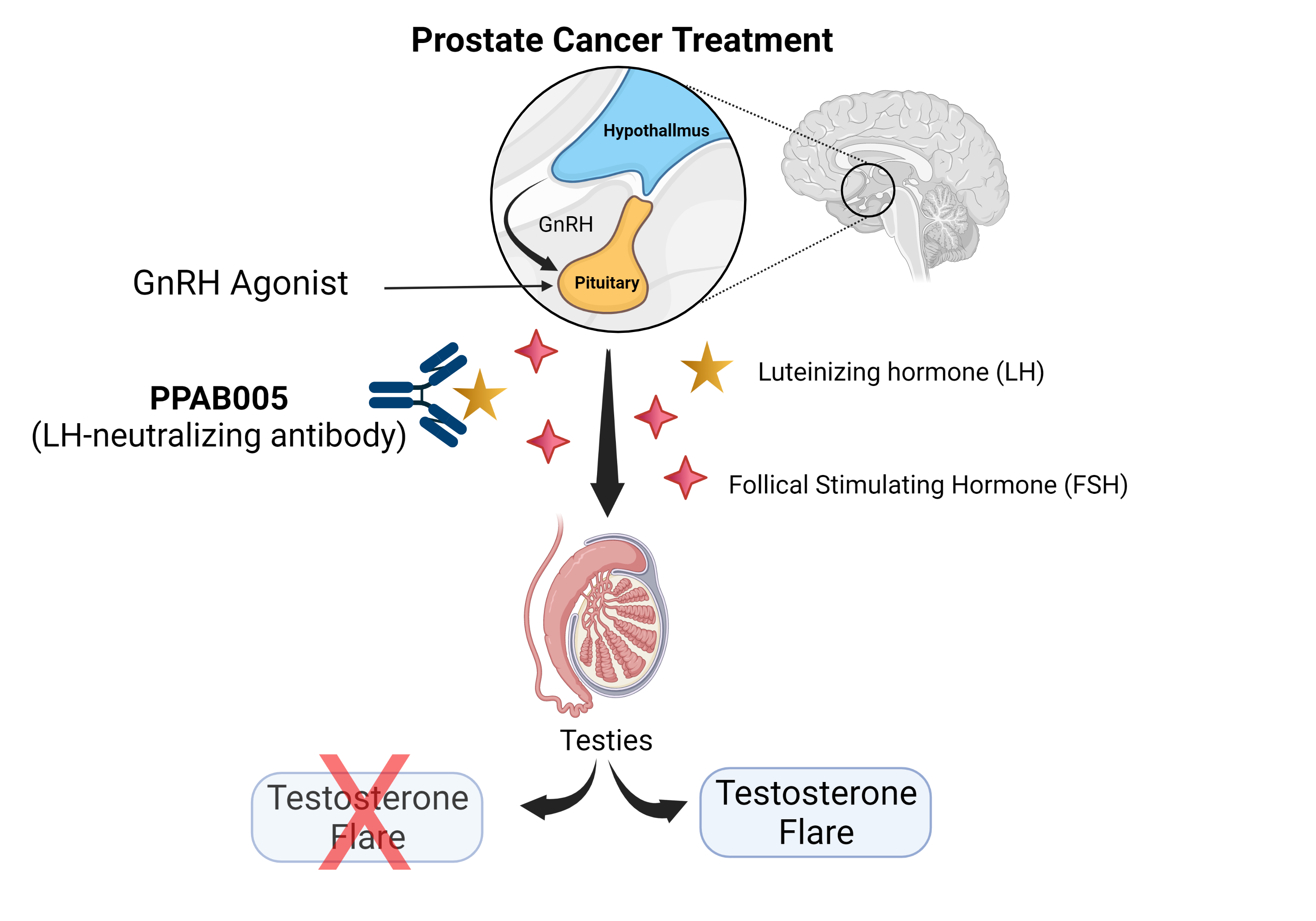
Androgen deprivation therapy (ADT) is the standard of care for treating advanced prostate cancer. ADT involves a continuous treatment of gonadotropin-releasing hormone (GnRH) agonists. GnRH agonists are synthetic proteins that are structurally similar to GnRH and bind to GnRH receptors in the pituitary gland.
When patients first receive a treatment with GnRH agonists, they usually experience a phenomenon called “testosterone flare” where GnRH agonists briefly cause the pituitary gland to secrete extra luteinizing hormone (LH) before blocking its release. The flare may worsen clinical symptoms by causing unwanted side effects such as bone pain, ureter or bladder outlet obstruction, and spinal cord compression.
To overcome the side effects of this therapeutic approach, we have developed a panel of LH- neutralizing antibodies (denoted as PPAB005) to counter the testosterone stimulation effects caused by LH.